
With a simple text prompt, it feels like magic.
Using generative AI tools like ChatGPT and Midjourney, we have results in seconds — incredible images, or even video, and functional software code.
Even a child can easily interface with a generative AI.
So it’s no surprise that most of the attention and excitement around generative artificial intelligence (AI) right now centers around the ability to generate text, images, designs, or software code.
It makes sense.
That’s not to downplay the importance of this technology, though.
One way that we can think of generative AI technology is that it’s a way for a computing system — or an AI — to communicate with us in our natural language. Any language in fact.
What doesn’t get as much press, though, are the more challenging applications of generative AI — like solving complex problems or coming up with creative solutions that would otherwise be unlikely to be discovered.
We explored this kind of application in Outer Limits — DeepMind’s Latest Breakthrough, when AI was used to generate 2.2 million new synthetic materials. Specifically crystals — some of which are capable of powering a new wave of technological development.
And again in Outer Limits — The Technology That Will Change the Economics of Biotech. That came after a promising private biotech company — Insilico — used generative AI to develop hundreds of new compounds for a novel new drug… and later identified 78 for further testing in the lab.
Results were remarkable, as I wrote back in March:
“In Insilico’s case, researchers were able to use generative AI to go from target discovery to pre-clinical candidate nomination in just 18 months at a cost of only $2.6 million. This is a process that normally takes about 5 years and tens of millions of dollars… with extremely high rates of failure.”
One of the compounds, known as molecule 055, showed remarkable characteristics for further drug development… and is now undergoing further testing in a human trial.
None of this would be possible without generative AI.
The power of this technology is that — as long as there are large enough data sets to learn from — AI can be used to generate any kind of output desired by the user.
And one of the most data-rich sectors that will benefit from this technology is life sciences and biotechnology.
We’re just at the very beginning of what’s possible with generative AI.
We’re going to see a new breed of artificial intelligence company — one that harnesses data that can be leveraged by the biopharmaceutical industry.
A good example of this emerging trend is Profluent Bio, a biotech-centric tech company that was founded less than two years ago.
The company generated some excitement in the last two weeks with the announcement of OpenCRISPR, an open-source, generative AI… designed to create a wide variety of proteins, including those used in genetic editing.
As a reminder, CRISPR technology is a revolutionary biotechnology that enables the editing of human, animal, and plant DNA. The technology empowers us to correct mutations in our DNA that cause disease, much in the way that we can correct bugs in software.
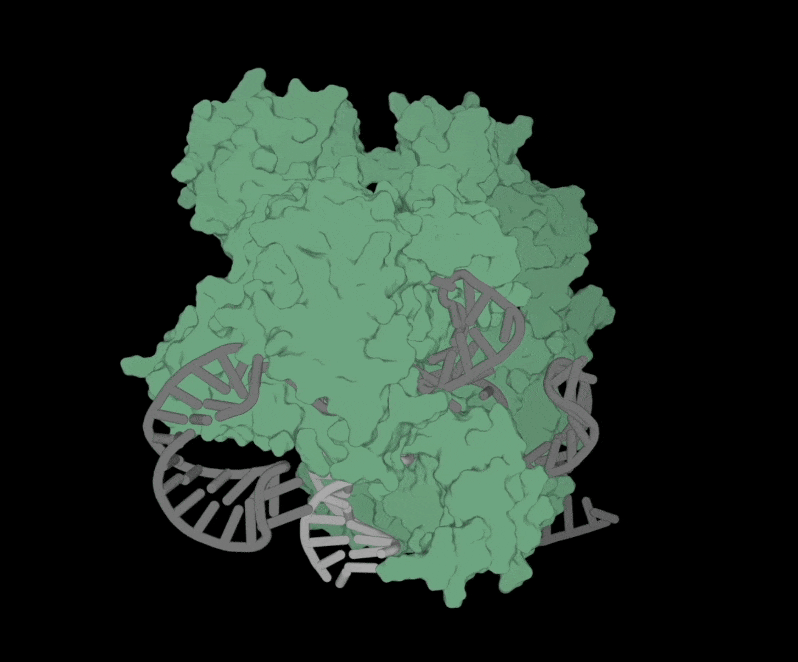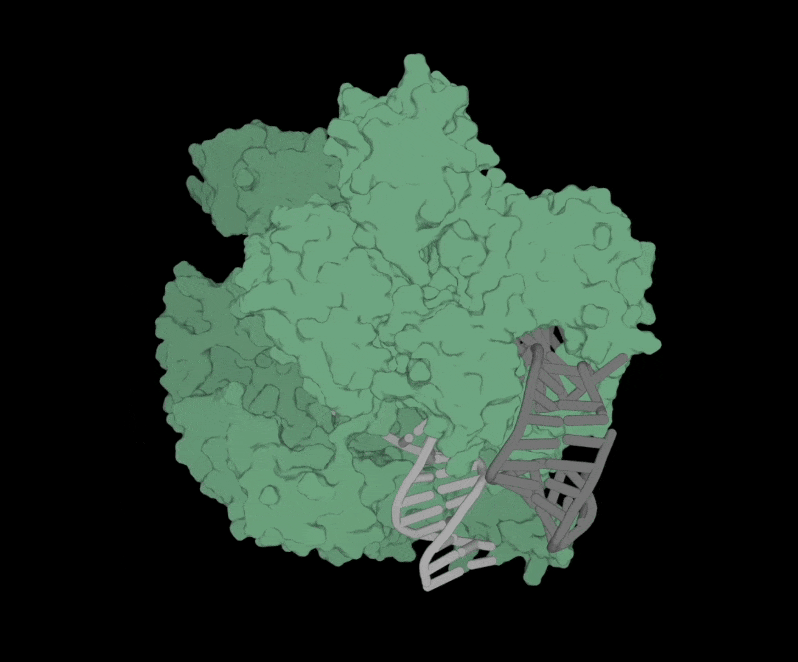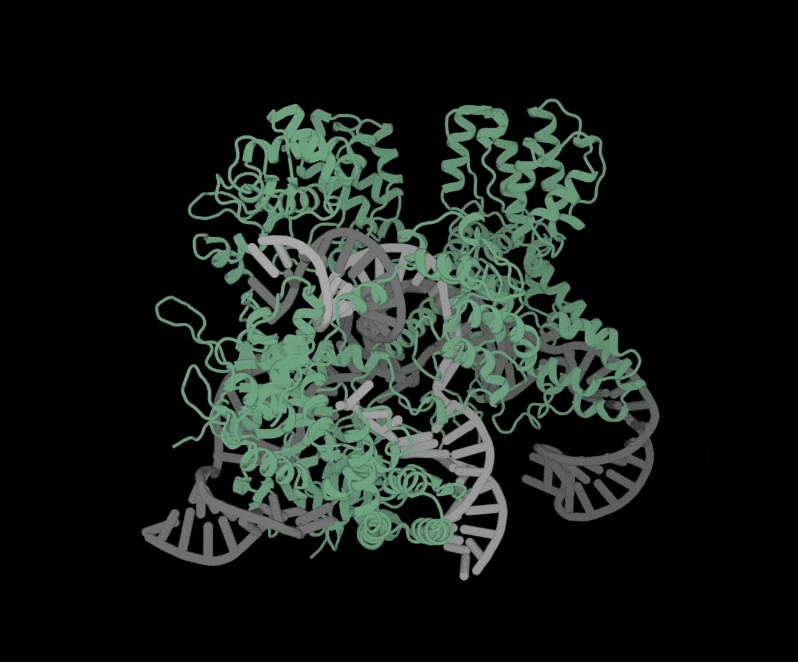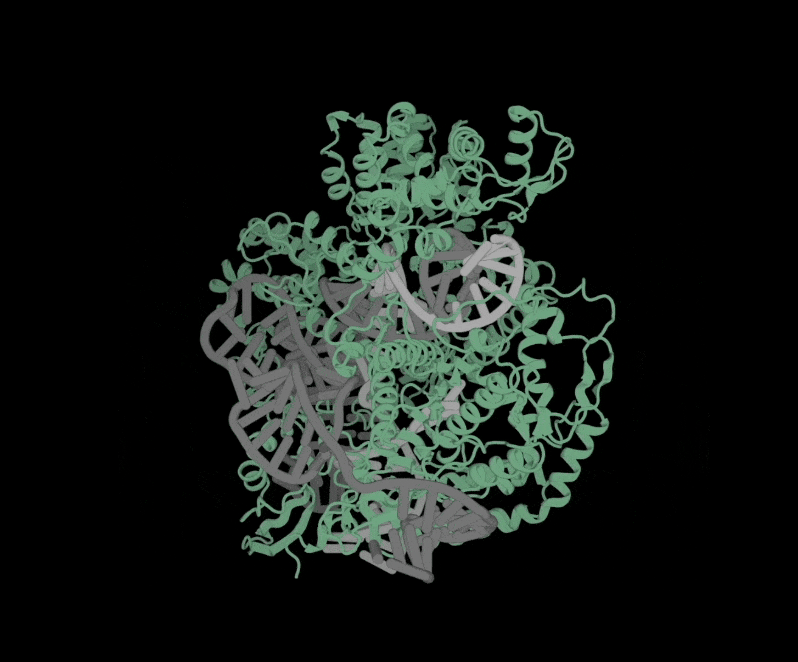
At a high level, a genetic therapy is delivered to human cells in an enzyme (in gray) using a guide RNA (shown in orange) that guides the genetic therapy to the target location in our DNA.
Attached to the guide RNA is the therapeutic payload (shown in yellow) — the correct DNA sequence.
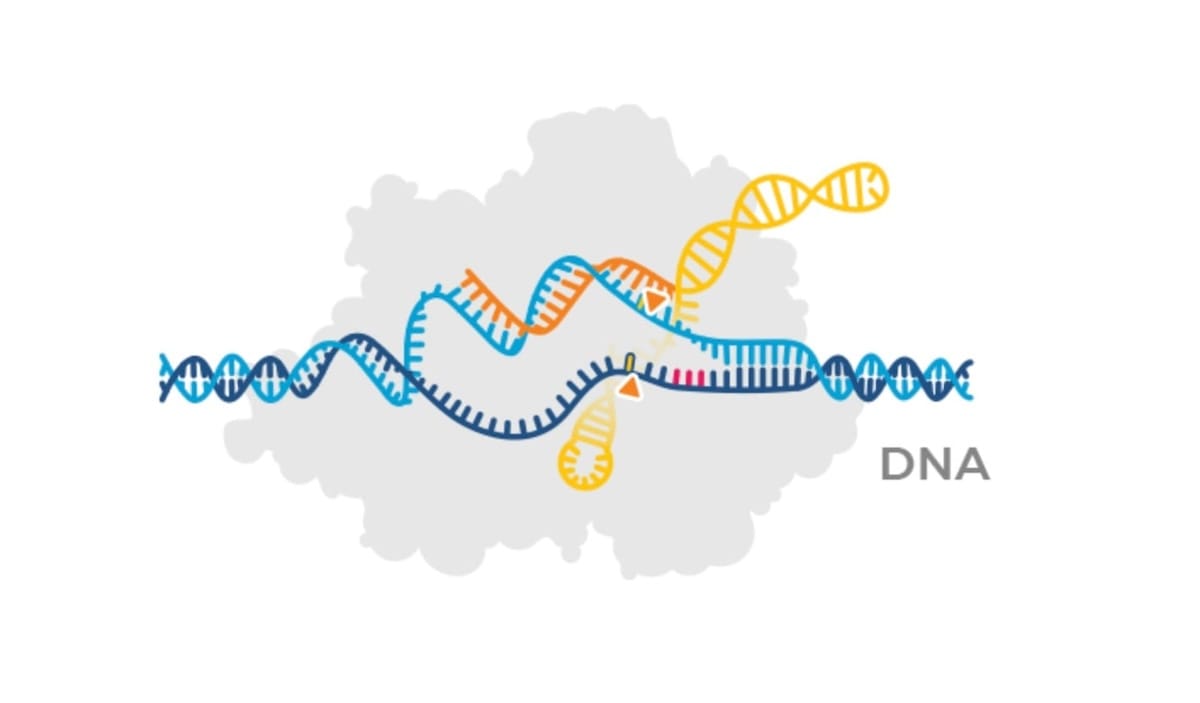
The enzyme (gray) facilitates the cutting or editing of the DNA, enabling the correct DNA sequence to be inserted or edited into the existing DNA. It’s an elegant solution to a complex problem that has given birth to a future where all human disease caused by unwanted genetic mutations can be managed or cured.
OpenCRISPR is interesting because its AI is trained to develop millions of CRISPR-like proteins (enzymes) that do not exist in nature. Said another way, the AI designs synthetic proteins.
Structure of OpenCRISPR-1 Complex
The software is free to use and accessible to anyone. All they have to do is fill out a short form with contact information and intended use of the software.
After that, users have to sign a license agreement, stating that they will only use the software for ethical purposes.
I’m sure that some of us are wondering, if Profluent is giving access to this powerful technology for free, and users can even commercialize the protein designs, how can Profluent survive?
What’s the business model?
Profluent’s business model is an interesting dynamic. Because Profluent’s announcement is marketed and written about as the open sourcing of its code is a magnanimous gift to the world.
And yet the motivations are entirely business driven.
Profluent’s actual business is to create and sell (or license) proteins for the biopharmaceutical industry. And the company is not giving away the source code for its artificial intelligence.
Instead, users can use the software free, but they can’t access and edit the software for their own purposes.
The underlying goal of making OpenCRISPR free for use is to accelerate the development of the software and interesting protein discoveries. In that way, this open-source release is designed to basically crowdsource software improvement and protein creation.
Better yet, part of the licensing agreement requires users to provide information back to Profluent — including which proteins they intend to bring to clinical trials and commercialize. This is invaluable information that costs Profluent nothing at all.
Profluent has only raised $44 million to date, so the way that the team has structured the open-source license agreement is advantageous to Profluent. It’s basically a way to get free labor, which will help the company with its technology development.
In order to get the industry excited, Profluent concurrently released research that demonstrated the successful genetic editing of human cells using a newly designed protein.
Human Cells Being Edited by OpenCRISPR-1 Designed Protein
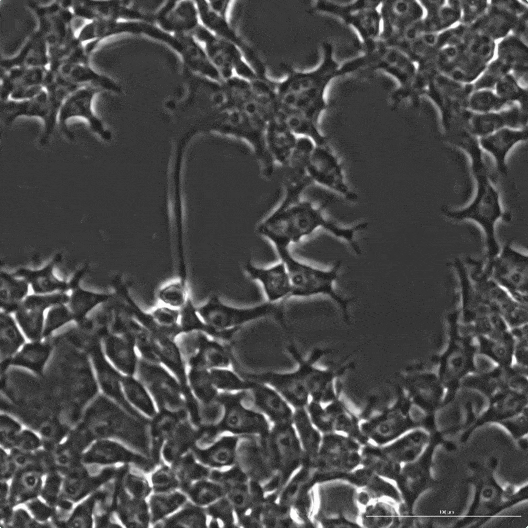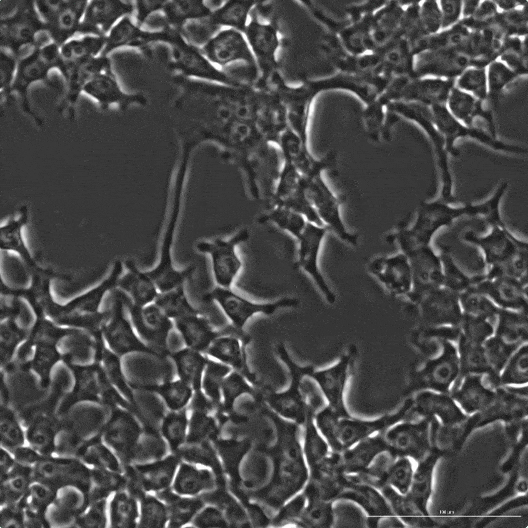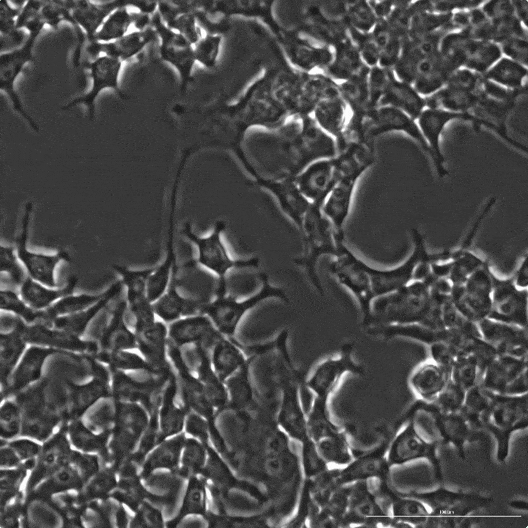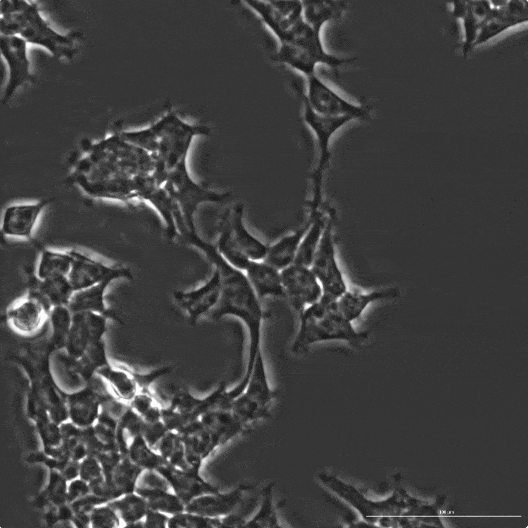
The research is encouraging. And it’s also important to keep in context.
This was just a laboratory test using human cells and a newly created synthetic protein. While the new synthetic protein demonstrated “comparable” on-target editing relative to the Cas9 protein (CRISPR associated protein 9), it’s a very long way from a clinical trial.
The release of OpenCRISPR also raises some interesting industry questions, including some about intellectual property and licensing of patents.
OpenCRISPR simply empowers users to create proteins for use with CRISPR technology.
It doesn’t absolve those users or companies from paying license fees for the use of CRISPR technology.
Here’s an important insight we must all keep in mind…
OpenCRISPR-1 (version 1) maintains the prototypical architecture of Cas9 proteins…
In other words, the AI was trained on Cas9 proteins and related CRISPR systems. And therefore creates Cas9-like synthetic proteins.
And this is precisely where Editas Medicine (EDIT) has such a strong intellectual property foothold.
In fact, Editas Medicine already has 74 related U.S. patents with an additional 54 pending.
Additionally, it has 35 European patents with 31 more pending. Editas has additional international patents, as well. They relate not just to Cas9, but Cas12a, as well as the underlying CRISPR technology.
As mentioned before, synthetic proteins created by an AI are those not found in nature. They thus will have to undergo extensive safety testing during the clinical process.
It is highly likely that it will be several years before we see one of these proteins used in Phase 3 clinical trials, let alone receive an FDA approval.
And it is only the point of commercialization where biopharmaceutical companies are on the hook for paying royalties for the use of intellectual property.
That process has already started, as Vertex Pharmaceuticals has already taken out a license from Editas Medicine for its jointly developed drug with CRISPR Therapeutics, Casgevy. We covered this in Outer Limits — A Resounding Victory in Biotech.
Vor Bio followed quickly with a non-exclusive license from Editas for CRISPR-Cas9-related patents, for the use in the treatment and prevention of hematological malignancies.
While Editas has a wide range of intellectual property, Cas9 itself is widely used in the genetic editing industry for its incredible utility. It can accurately target just about any place in the human genome. It also has a compact form, making it ideal for a genetic therapy which needs to enter human cells.
Editas estimates that CRISPR-Cas9 and CRISPR-Cas12a have the ability to enable genetic editing of 95% of the human genome.
It’s hard to grasp the significance of this incredible tool — other than to acknowledge the ability to remove unwanted mutations or suppress genes that create unwanted proteins — in order to cure all genetically caused human disease.
We can think of Profluent’s open-source offering as an accelerant. Not just to Profluent’s own business and tech development, but also for the genetic editing industry at large.
We always welcome your feedback. We read every email and address the most common comments and questions in the Friday AMA. Please write to us here.